Professor Jie-Peng Zhang’s group: “Graphene-Like Hydrogen-Bonded Melamine-Cyanuric Acid Supramolecular Nanosheets as Pseudo-Porous Catalyst Support”
Source: School of Chemistry
Edited by: Tan Rongyu, Wang Dongmei
Nanoparticles (NPs) of metal oxides, phosphides, carbides, and nitrides have been extensively used as heterogeneous catalysts. Because of the instability associated with ultra-small sizes and high surface energy, NPs are suffering from aggregation during catalytic reactions, resulting in deactivation and poor durability. The most convenient way to solve this problem is immobilization of NPs onto a suitable support/carrier. Meanwhile, the interaction between supports and NPs can also modulate the electronic structure of the catalysts, which can improve the catalytic activity. Therefore, the development of new catalyst supports is one of the important researches in heterogeneous catalysis in recent years.
Besides high surface area, chemical stability is another important characteristic of catalyst supports. Therefore, the commonly used catalyst supports are based on covalent bonds, such as graphene and its derivatives. When 2D materials and/or ultrathin nanosheets are used as catalyst supports, NPs generally contact only with the surfaces of the 2D/nanosheet supports, which is easy to fall off during the catalytic process. In principle, embedding the NPs in the pores of porous supports can provide a better contact. However, the robust covalent bonds in these supports make them hard to form pores. Porous coordination polymers/metal-organic frameworks have attracted much attention as new types of catalyst supports in recent years. However, such materials based on coordination bonds are hard to work in harsh conditions. Hydrogen bonds are noncovalent interaction even weaker than coordination bonds, and the corresponding supramolecular materials are more unstable, so it seems difficult to be used as catalyst supports.
Recently, Professor Jie-Peng Zhang’s group (School of Chemistry, Sun Yat-sen University) and professor Chun-Ting He (College of Chemistry and Chemical Engineering, Jiangxi Normal University) report hydrogen-bonded ultrathin nanosheets as a new type of catalyst supports. The classic hydrogen-bonded co-crystal M-CA can be easily exfoliated by ultrasonic treatment to yield ultrathin nanosheets with thickness of
ca. 1.6 nm and high stability at pH = 0. The dynamic nanosheets form adaptive defects/pores in the synthetic process of CoP NPs, giving composite catalyst denoted as CoP@M-CA.

Figure 1. Typical composite types between nanoparticles and nanosheets. a) NPs attached on the surfaces of nonporous supports. b) NPs embedded in the pores of porous supports. c) NPs embedded in the in-situ formed defects of nonporous supports. Graphene, porous graphene, and M-CA nanosheets are shown as example 2D supports.
The HER activity of CoP@M-CA (66 mV at 10 mA cm–2) in 0.5 M H
2SO
4 is higher than that of not only CoP (275 mV) but also CoP@G (133 mV). Moreover, the potential only increases by 6 mV after chronopotentiometry at 10 mA cm–2 for 15 h. Computational calculations, X-ray photoelectron spectroscopy and X-ray absorption spectroscopy unveiled that some electrons are transferred from CoP to M-CA and the values of the free energy for H* intermediate are optimized, meaning that M-CA ultrathin nanosheets can not only immobilize and protect but also modulate the electron structure of the CoP catalyst. This work demonstrates the potential application of hydrogen-bonded supramolecular compounds in heterogeneous catalysis.
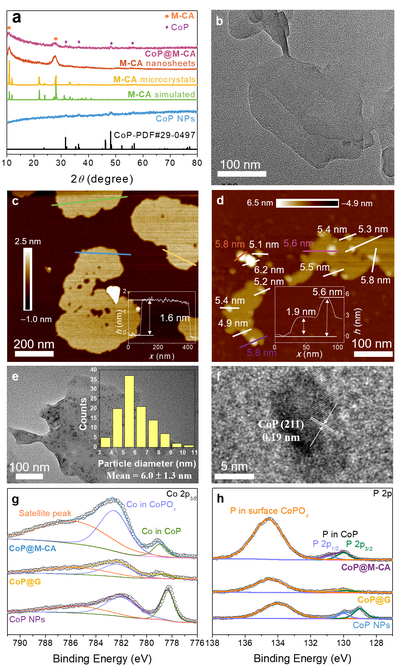
Figure 2. Structural characterization of the nanosheets and catalysts. a) PXRD patterns of the as-synthesized samples of M-CA microcrystals, M-CA nanosheets, CoP NPs, and CoP@M-CA. b) TEM image of M-CA nanosheets. c) AFM image of M-CA nanosheets (inset: corresponding height profile along the green line). d) AFM image of CoP@M-CA (inset: corresponding height profile along the pink line). e) TEM image of CoP@M-CA (inset: particle size distribution of CoP NPs). f) HRTEM image of a typical CoP nanoparticle in CoP@M-CA. High resolution XPS spectra of g) Co 2p3/2 and h) P 2p for CoP NPs, CoP@G and CoP@M-CA.
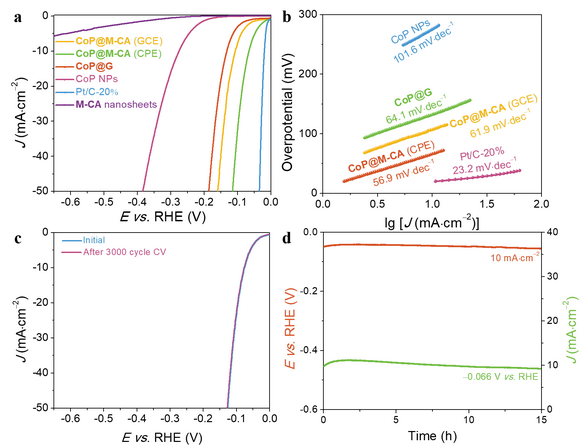
Figure 3. HER performances of the composite catalyst. a) LSV curves of CoP@M-CA and relevant materials. b) Tafel plots of CoP@M-CA and relevant materials. c) LSV curves of CoP@M-CA after 3000 cyclic voltammetry scans. d) chronoamperometry and chronopotentiometry curves. All electrochemical measurements were carried out in 0.5 M H
2SO
4.
This work is recently published in
Advanced Materials. Professor Jie-Peng Zhang (Sun Yat-sen University) and Professor Chun-Ting He (Jiangxi Normal University) are the corresponding authors. PhD candidate Jun-Xi Wu and post-doctoral Partha Pratim Bag contribute equally to this work. This work was supported by the National Natural Science Foundation of China (21821003, 21975290, 21890380, and 21901089), the Guangdong Pearl River Talents Program (2017BT01C161), and the Foundation of Basic and Applied Basic Research of Guangdong Province (2020B1515120024). C.-T.H. acknowledges the Jiangxi Province (20202ZDB01004 and jxsq2018106041).
Link to the paper:
https://onlinelibrary.wiley.com/doi/10.1002/adma.202007368
