Recently, Prof. Kuang Ming's team (Center for Hepato-Pancreato-Biliary Surgery, The First Affiliated Hospital, Sun Yat-sen University) and Prof. Lin Shuibin's team (Institute of Precision Medicine, The First Affiliated Hospital, Sun Yat-sen University) co-published a research article entitled “Targeting tumor-intrinsic N7-methylguanosine tRNA modification inhibits MDSC recruitment and improves anti-PD-1 efficacy”. It was published in Gut, a top journal in the field of gastroenterology. This study reveals the cellular and molecular basis of crosstalk between ICC cells and MDSCs in shaping TIME and affecting ICIs efficacy, which enables reasonable design of new combined therapy targeting dysregulated mRNA translation and suppressive TIME.
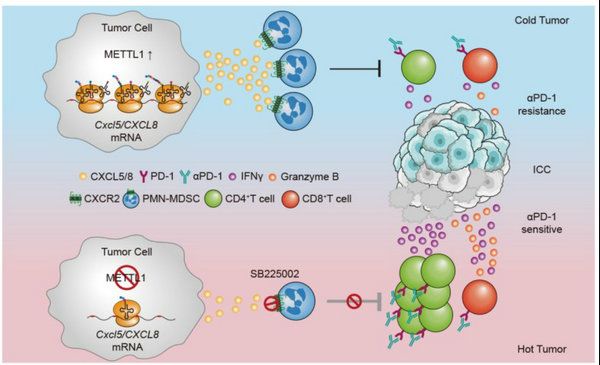
Tumor-intrinsic METTL1 regulate the translation of CXCL8/Cxcl5 to recruit PMN-MDSC which further lead to anti-PD-1 resistance by inhibiting the CD4+ T cell expansion and the antitumor activity of CD4+ and CD8+ T cells.
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary liver cancer. 78% of ICC patients are initially diagnosed with advanced disease and have limited treatment options, leading to dismal prognosis. Unfortunately, the ICIs treatment efficacy in ICC patients is very poor, the response rate in ICC is much lower than other solid tumors. Therefore, further understanding the underlying mechanism of ICC progression and developing new effective therapeutic strategies are urgently needed.
Accumulating studies have revealed that a large population of myeloid-derived suppressor cells (MDSCs) are infiltrated in tumor immune microenvironment (TIME), which have a close link to the clinical efficacy of ICIs therapy and rapid tumor progression. Based on the liver cancer database in the First Affiliated Hospital, Sun Yat-sen University, Prof. Kuang Ming’s team found that Polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs) are enriched in advanced ICCs and significantly correlated with N7-methylguanosine (m7G) tRNA methyltransferase METTL1. METTL1 regulated the accumulation of PMN-MDSC in TIME and ICC progression. Mechanistically, CXCL8 in human and Cxcl5 in mouse are key translational targets of METTL1 that facilitate its function in promoting PMN-MDSC accumulation in TIME and ICC progression in vivo. Co-blockade of METTL1 and its downstream chemokine pathway enhances the anti-PD-1 efficacy in ICC pre-clinical mouse models.
The first authors of this paper are Ph.D. student Liu Haining, Dr. Zeng Xuezhen and Ph.D. student Ren Xuxin, from the Center for Hepato-Pancreato-Biliary Surgery. The corresponding author is Prof. Kuang Ming and Prof. Lin Shuibin. Prof. Kuang Ming and Prof. Lin Shuibin have cooperated for a long time in the field of abnormal mRNA translation on liver cancer. They have revealed the key role of abnormal mRNA translation in the initiation and development of liver cancer.
Link to the paper:https://gut.bmj.com/content/early/2022/10/25/gutjnl-2022-327230



